Edelgase
Eine Familie von edlem Gemüt

The Chemistry of Noble Gases

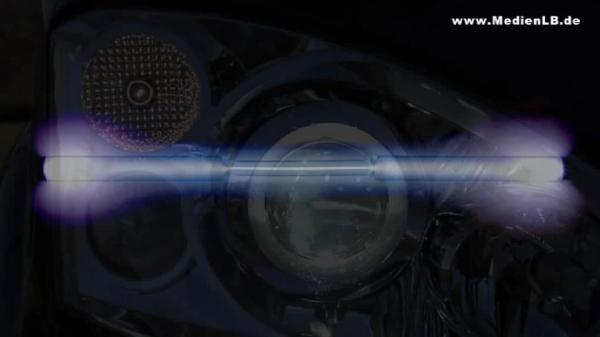
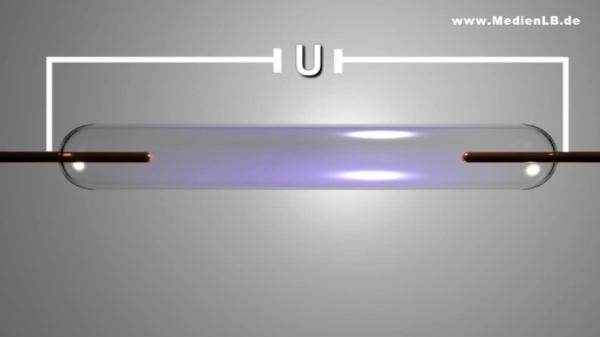
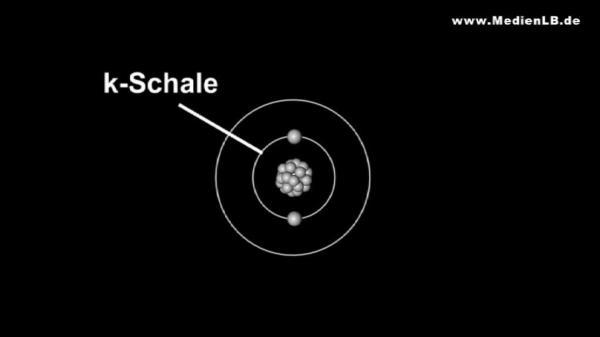
Dusk is falling – time for car drivers to switch on their headlights. If you look around a bit, some cars catch your eye because of their bluish light. They are equipped with a special generation of car lamps – with xenon lights. They are called like that because they contain the gas xenon that is responsible for their bluish colour. Xenon is a noble gas and, as such, a member of group 8 of the periodic table of the elements. Besides xenon, there are the noble gases helium, neon, argon, krypton and the radioactive radon. Why are these gases called noble gases? You certainly remember the noble metals you learnt about in your chemistry lessons. Metals like gold or platinum are hardly corroded by other substances such as acids. As they show little tendency to react with oxygen compared with less noble metals, we find them in their pure, that is metallic, form in nature. What have these noble metals in common with the noble gases? The "noble" in their name is no indication that they are particularly precious elements and does not mean "sheer" or "pure". In chemistry it stands for "hardly reactive". The noble gases are an element family whose members are particularly inert. In nature, no compounds of noble gases are known and for a long time it was believed that they are completely unable to react chemically with other elements. Only in 1962, the first compounds with noble gases were created artificially. It turned out, however, that only the heavy representatives of the noble gases such as xenon or krypton are capable to react chemically with the halogens or with oxygen. Then, in the year 2000, the first compound with argon was produced. All noble gases are colourless and odourless, nonflammable and nontoxic. Contrary to the other elements that are gaseous under standard conditions the noble gases do not form diatomic molecules but occur in an atomic state. Radon is radioactive and is one of the rarest elements we know. Therefore, we will disregard it in our subsequent considerations.